Which Of The Following Shows The Correct Makeup Of Human Chromosomes
Each chromosome pair viewed in a karyotype appears to take its ain distinct "bar code" of bands. What changes do scientists await for in a karyotype when diagnosing diseases and disorders?
Karyotyping is the process of pairing and ordering all the chromosomes of an organism, thus providing a genome-wide snapshot of an individual's chromosomes. Karyotypes are prepared using standardized staining procedures that reveal characteristic structural features for each chromosome. Clinical cytogeneticists analyze human karyotypes to detect gross genetic changes—anomalies involving several megabases or more of DNA. Karyotypes tin reveal changes in chromosome number associated with aneuploid conditions, such equally trisomy 21 (Downwards syndrome). Careful assay of karyotypes can also reveal more subtle structural changes, such equally chromosomal deletions, duplications, translocations, or inversions. In fact, as medical genetics becomes increasingly integrated with clinical medicine, karyotypes are becoming a source of diagnostic information for specific birth defects, genetic disorders, and even cancers.
Preparing Karyotypes from Mitotic Cells
Karyotypes are prepared from mitotic cells that accept been arrested in the metaphase or prometaphase portion of the cell cycle, when chromosomes assume their virtually condensed conformations. A diverseness of tissue types can exist used as a source of these cells. For cancer diagnoses, typical specimens include tumor biopsies or os marrow samples. For other diagnoses, karyotypes are ofttimes generated from peripheral claret specimens or a pare biopsy. For prenatal diagnosis, amniotic fluid or chorionic villus specimens are used every bit the source of cells.
The process of generating a karyotype begins with the short-term culture of cells derived from a specimen. Afterward a period of cell growth and multiplication, dividing cells are arrested in metaphase past addition of colchicine, which poisons the mitotic spindle. The cells are next treated with a hypotonic solution that causes their nuclei to cracking and the cells to burst. The nuclei are then treated with a chemical fixative, dropped on a drinking glass slide, and treated with various stains that reveal structural features of the chromosomes.
Banding Patterns Reveal the Structural Details of Chromosomes
Without any treatment, structural details of chromosomes are difficult to discover under a light microscope. Thus, to brand analysis more effective and efficient, cytologists take developed stains that bind with DNA and generate characteristic banding patterns for different chromosomes. Prior to the development of these banding techniques, distinguishing chromosomes from one another proved very difficult, and chromosomes were only grouped co-ordinate to their size and the placement of their centromeres.
This changed in 1970, when Torbjorn Caspersson and his colleagues described the showtime banding technique, known every bit Q-banding. Q-banding involves use of the fluorescent dye quinacrine, which alkylates DNA and is subject to quenching over time. Caspersson et al. demonstrated that quinacrine produced characteristic and reproducible banding patterns for individual chromosomes. Since and so, researchers take adult a variety of other chromosome banding techniques that take largely supplanted Q-banding in clinical cytogenetics. Today, most karyotypes are stained with Giemsa dye, which offers better resolution of individual bands, produces a more stable preparation, and can be analyzed with ordinary brilliant-field microscopy.
The molecular causes for staining differences along the length of a chromosome are complex and include the base composition of the DNA and local differences in chromatin construction. In M-banding, the variant of Giemsa staining virtually commonly used in North America, metaphase chromosomes are first treated briefly with trypsin, an enzyme that degrades proteins, before the chromosomes are stained with Giemsa. Trypsin partially digests some of the chromosomal proteins, thereby relaxing the chromatin structure and allowing the Giemsa dye access to the DNA.
In full general, heterochromatic regions, which tend to be AT-rich DNA and relatively factor-poor, stain more darkly in Grand-banding. In contrast, less condensed chromatin—which tends to be GC-rich and more transcriptionally active—incorporates less Giemsa stain, and these regions announced equally calorie-free bands in M-banding. Almost importantly, G-banding produces reproducible patterns for each chromosome, and these patterns are shared between the individuals of a species. An example of Giemsa-stained human chromosomes, as they would appear under a microscope, is shown in Figure 1a. Typically, Giemsa staining produces between 400 and 800 bands distributed amid the 23 pairs of human chromosomes. Measured in Deoxyribonucleic acid terms, a Thousand-band represents several million to x million base pairs of DNA, a stretch long enough to contain hundreds of genes.
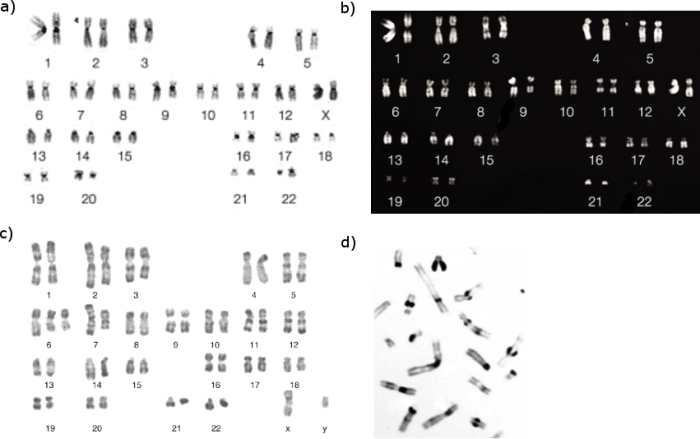
Effigy 1: Chromosome banding revealed by different staining techniques.
Different chromosomal staining techniques reveal variations in chromosome construction. Cytogeneticists use these patterns to recognize the differences between chromosomes and enable them to link different affliction phenotypes to chromosomal abnormalities. Giemsa banding (a), Q-banding (b), R-banding (c) and C-banding (d) are shown.
© 2001 Nature Publishing Group Rowley, J. Chromosome translocations. Nature Reviews Cancer 1, 246; Stamatoullas, A. et al. Conventional cytogenetics of nodular lymphocyte-predominant Hodgkin's lymphoma. Leukemia 21, 2065; Vega, H. et al. Roberts syndrome is caused by mutations in ESCO2, a human homolog of yeast ECO1 that is essential for the institution of sister chromatid cohesion. Nature Genetics 35, 469 (2001). All rights reserved. ![]()
G-banding is not the just technique used to stain chromosomes, however. R-banding, which is used in parts of Europe, too involves Giemsa stain, but the procedure generates the reverse pattern from Grand-banding. In R-banding (Figure 1c), the chromosomes are heated before Giemsa stain is practical. The heat treatment is idea to preferentially cook the Dna helix in the AT-rich regions that normally bind Giemsa stain most strongly, leaving only the comparatively GC-rich regions to accept up the stain. R-banding is frequently used to provide critical details about gene-rich regions that are located near the telomeres.
Notwithstanding another method is C-banding (Figure 1d), which can exist used to specifically stain constitutive heterochromatin, or genetically inactive Deoxyribonucleic acid, just information technology is rarely used for diagnostic purposes these days. C-banding is a specialized Giemsa technique that primarily stains chromosomes at the centromeres, which accept large amounts of AT-rich satellite DNA.
The commencement method to be used to identify all 46 homo chromosomes was Q-banding (Figure 1b), which is accomplished past staining the chromosomes with quinacrine and examining them under UV light. This method is most useful for examining chromosomal translocations, especially ones involving the Y chromosome. Taken together, these banding techniques offer clinical cytogeneticists an arsenal of staining methods for diagnosing chromosomal abnormalities in patients.
Organizing Chromosomes in Karyograms for Review
In club to maximize the diagnostic information obtained from a chromosome preparation, images of the private chromosomes are bundled into a standardized format known every bit a karyotype, or more precisely, a karyogram (Figure 1a-c). Co-ordinate to international conventions, human autosomes, or non-sex activity chromosomes, are numbered from i to 22, in descending order by size, with the exceptions of chromosomes 21 and 22, the former actually being the smallest autosome. The sexual activity chromosomes are generally placed at the stop of a karyogram.
Within a karyogram, chromosomes are aligned forth a horizontal centrality shared by their centromeres. Private chromosomes are e'er depicted with their short p arms—p for "petite," the French give-and-take for "small"—at the top, and their long q arms—q for "queue"—at the bottom. Centromere placement can also exist used to identify the gross morphology, or shape, of chromosomes. For example, metacentric chromosomes, such equally chromosomes ane, 3, and 16, have p and q arms of nearly equal lengths. Submetacentric chromosomes, such as chromosomes two, 6, and x, take centromeres slightly displaced from the heart. Acrocentric chromosomes, such as chromosomes 14, 15, and 21, accept centromeres located near their ends.
Arranging chromosomes into a karyogram can simplify the identification of whatever abnormalities. Notation that the banding patterns between the two chromosome copies, or homologues, of any autosome are nearly identical. Some subtle differences between the homologues of a given chromosome can be attributed to natural structural variability amid individuals. Occasionally, technical artifacts associated with the processing of chromosomes will also generate apparent differences between the two homologues, but these artifacts tin exist identified by analyzing 15–20 metaphase spreads from ane individual. It is highly unlikely that the same technical artifact would occur repeatedly in a given specimen.
Using Karyograms to Detect Chromosomal Abnormalities
Today, One thousand-banded karyograms are routinely used to diagnose a wide range of chromosomal abnormalities in individuals. Although the resolution of chromosomal changes detectable by karyotyping is typically a few megabases, this tin be sufficient to diagnose certain categories of abnormalities. For example, aneuploidy, which is often caused by the absence or improver of a chromosome, is simple to detect by karyotype analysis. Cytogeneticists can also often detect much more than subtle deletions or insertions as deviations from normal banding patterns. Too, translocations are ofttimes readily apparent on karyotypes.
When regional changes in chromosomes are observed on karyotypes, researchers often are interested in identifying candidate genes within the critical interval whose misexpression may cause symptoms in patients. This search process has been greatly facilitated by the completion of the Homo Genome Project, which has correlated cytogenetic bands with Dna sequence information. Consequently, investigators are now able to utilise a range of molecular cytogenetic techniques to reach even higher resolution of genomic changes. Fluorescence in situ hybridization (FISH) and comparative genomic hybridization (CGH) are examples of two approaches that can potentially identify abnormalities at the level of private genes.
Molecular cytogenetics is a dynamic discipline, and new diagnostic methods proceed to be adult. As these new technologies are implemented in the dispensary, nosotros can wait that cytogeneticists will exist able to brand the leap from karyotype to cistron with increasing efficiency.
References and Recommended Reading
Caspersson, T., Zech, L., & Johansson, J. Differential banding of alkylating fluorochromes in human chromosomes. Experimental Cell Research 60, 315–319 (1970) doi:ten.1016/0014-4827(70)90523-nine
Gartler, S. Chiliad. The chromosome number in humans: A brief history. Nature Reviews Genetics 7, 655–660 (2006) doi:x.1038/nrg1917 (link to article)
Speicher, Grand. R., Ballard, S. M., & Ward, D. C. Karyotyping human chromosomes by combinatorial multi-fluor FISH. Nature Genetics 12, 368–375 (1996) (link to article)
Strachan, T., & Read, A. P. Homo Molecular Genetics, 2nd ed. (Wiley, New York, 1999)
Tjio, J. H., & Levan, A. The chromosome number of homo. Hereditas 42, 1–6 (1956)
Trask, B .J. Human cytogenetics: 46 chromosomes, 46 years and counting. Nature Reviews Genetics iii, 769–778 (2002) doi:10.1038/nrg905 (link to commodity)
Source: http://www.nature.com/scitable/topicpage/karyotyping-for-chromosomal-abnormalities-298
Posted by: paigewilier88.blogspot.com

0 Response to "Which Of The Following Shows The Correct Makeup Of Human Chromosomes"
Post a Comment